
Campaigns, Policy Updates
The National Women’s Health Network’s 2024 Policy Agenda
January 24, 2024
---
Consumer Health Info
Publication Date: August 24, 2022
By: Sophie Krensky, Registered Nurse

 What is Phexxi?
What is Phexxi? On May 22, 2020, the U.S. Food and Drug Administration (FDA) approved a new contraceptive method, “Phexxi,” expanding the number of non-hormonal, on-demand options available to users who can become pregnant. Made by (and previously known as Amphora), Phexxi is a contraceptive in gel form that can be used instead of traditional spermicides.
Phexxi is a gel placed in the vagina up to an hour before penis-in-vagina sex. It works by taking advantage of the vagina’s natural acidity and sperm’s vulnerability to acidic environments. Phexxi only needs to be used before sex, unlike other methods that require daily or weekly action to stay protected against pregnancy. Phexxi does not contain hormones.
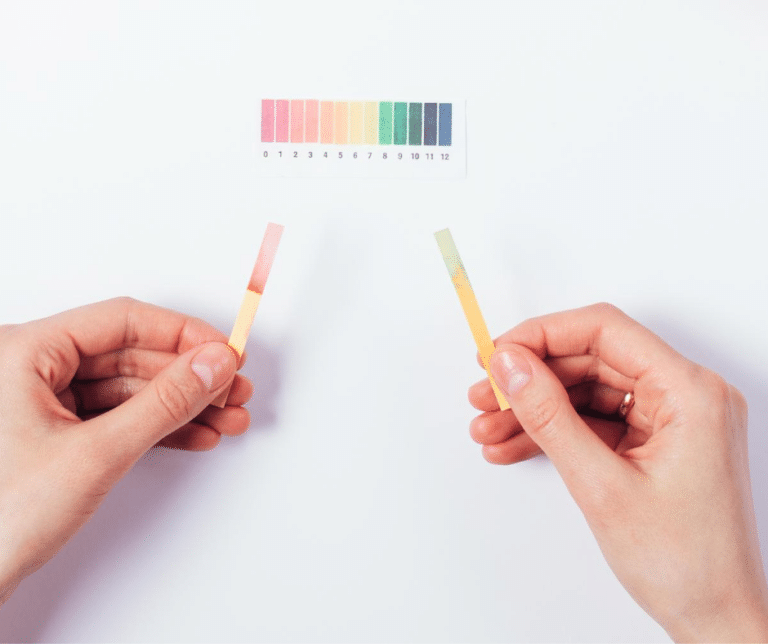
The pH scale measures how acidic or basic (alkaline) a substance is, with the most acidic substances registering as zero, the most basic substances registering as 14, and neutral substances registering as seven.
The natural pH of a healthy vagina ranges from 3.5 to 4.5 (moderately acidic), but during penis-in-vagina sex, semen (fluid that contains sperm, which has a slightly basic pH of 8) raises the vagina’s pH to 7 or 8 (neutral or mildly basic) to create an environment that is livable for the sperm, allowing for fertilization of an egg. Phexxi works by keeping the vaginal pH within the vagina’s pre-sex range, counteracting the influence of the semen and incapacitating the sperm so that it cannot reach the egg. The gel is comprised of lactic acid (which is naturally produced in the vagina and is found in dairy products like yogurt and kefir), citric acid (which gives citrus fruits their tart, sour taste), and potassium bitartrate (used in cooking as cream of tartar).
Phexxi comes in a box of 12 pre-filled applicators, each with a 5-gram dose. One dose is inserted into the vagina up to an hour before having penis-in-vagina sex. One dose is effective for each act of penis-in-vagina sex, so the user would need to insert another dose if they want to have sex again within one hour. Phexxi is not effective when used after sex. Phexxi can be used on its own or in conjunction with barrier methods such as condoms, cervical caps, and diaphragms for increased effectiveness. For a complete list of barrier methods and how to use them, see our article on “Vaginal Barrier Methods.”
Phexxi can be used any time during a person’s menstrual cycle and as soon as it is safe to have sex again after giving birth, having an abortion, or experiencing a miscarriage.
Phexxi is only approved for pregnancy prevention and is not currently approved to protect against sexually transmitted infections (STIs), including HIV, the virus that causes AIDS.
Like Phexxi, spermicide is inserted into the vagina immediately before penis-in-vagina sex and can be used in combination with barrier methods like condoms, cervical caps, and diaphragms. Spermicide comes in the form of cream, gel, foam, dissolvable film, and suppositories. In the U.S., the only FDA-approved spermicides contain nonoxynol-9 (N-9), a . While spermicide is safe for most people, N-9 can damage the cells in the vagina that protect against transmission of bacteria and viruses, particularly after repeated use. As a result, N-9 can lead to STI infections for sex workers and others who have sex multiple times per day. In contrast, Phexxi has a different mechanism of action than N-9 and is not expected to damage the vaginal wall.
For added pregnancy protection, Phexxi can be combined with other methods like oral contraceptive pills, condoms (internal or external), or an IUD. The FDA label cautions against the use of Phexxi in conjunction with vaginal rings.
Side effects (experienced by approximately 2% of participants in clinical trials) included vulvovaginal burning sensation, itching, yeast infection, discomfort, and pain; bacterial vaginosis (BV) and vaginal discharge; genital discomfort; and pain while urinating. More serious side effects (experienced by 0.36% of participants) included bladder inflammation, kidney infection, or another upper urinary tract infection (UTI).
These side effects are the reason that Phexxi is not recommended for people with a history of recurrent UTIs or urinary tract abnormalities. Dr. Jen Gunter, an OB-GYN and author of The Vagina Bible, points out that while Phexxi appears safe for those with average risk of bacterial vaginosis and UTIs, it may be several years until more robust, real-world data on BV and UTI incidence with Phexxi, much like other newly approved.
In one clinical trial for Phexxi, reported irritation to their partner’s penis while using Phexxi, such as itching, burning, and pain.

Phexxi, like many other prescription contraceptives, is covered by the Affordable Care Act (ACA) without co-pay or deductible, effective for those with plan years starting in 2023. Unlike N-9 containing spermicides, which are available over the counter, Phexxi requires a prescription from a healthcare provider. With health insurance, Phexxi will be low-cost or free; without health insurance, the price is likely to be about $250-$275 for 12 doses or $20-23 per dose.
